| Adverse reaction | COBENFY (n=251) |
Sugar pill (n=253) |
|---|---|---|
| Nausea | 19% | 4% |
| Stomach upset or burning (dyspepsia) |
18% | 5% |
| Constipation | 17% | 7% |
| Vomiting | 15% | 1% |
| High blood pressure | 11% | 2% |
| Stomach (abdominal) pain | 8% | 4% |
| Diarrhea | 6% | 2% |
| Increased heart rate | 5% | 2% |
| Dizziness | 5% | 2% |
| Heartburn (gastrointestinal reflux disease) |
5% | <1% |
Possible side effects
Kelsey is not taking COBENFY. Kelsey and Jia have been compensated for their time.
Most common side effects
These were experienced by at least 5% of patients taking COBENFY.
These are not all of the possible side effects of COBENFY. Your healthcare provider may lower your dose or stop treatment with COBENFY if you get certain side effects.
COBENFY may cause serious side effects, including:
Problems with emptying your bladder (urinary retention), risks in people with liver problems, risks in people with bile duct and gallbladder problems (biliary disease), slow emptying of your stomach (decreased gastrointestinal motility), serious allergic reactions (angioedema), an eye problem called narrow-angle glaucoma, increases in heart rate, side effects in people with kidney problems, and central nervous system problems.
Bryan is taking COBENFY. Bryan was compensated for his time.
First instance of nausea and vomiting often occurred during the first 2 weeks of treatment and generally decreased over time
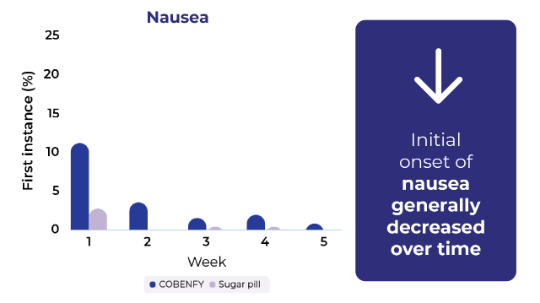
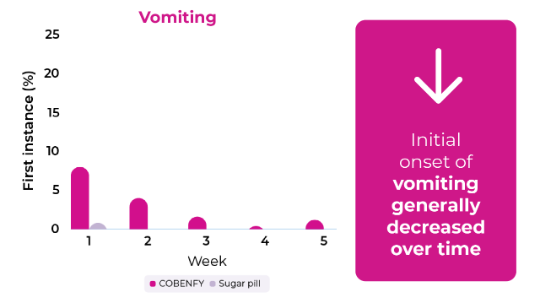

If you experience nausea or vomiting, ask your healthcare provider about options that may help.
Weight changes over time

| * | The results are from 1-year studies where participants and their physicians knew they were taking COBENFY, which may have influenced the results. |
| † | On average, patients lost 5.6 lbs in a year-long extension trial. 17.6% of patients had a decrease in body weight of ≥7%. 4.1% of patients had an increase in body weight of ≥7%. |
COBENFY and uncontrolled movements
People taking COBENFY had similar rates of uncontrolled facial or body movements (2%) as those taking a sugar pill (<1%).
Sagan is taking COBENFY. Sagan was compensated for his time.



